Description
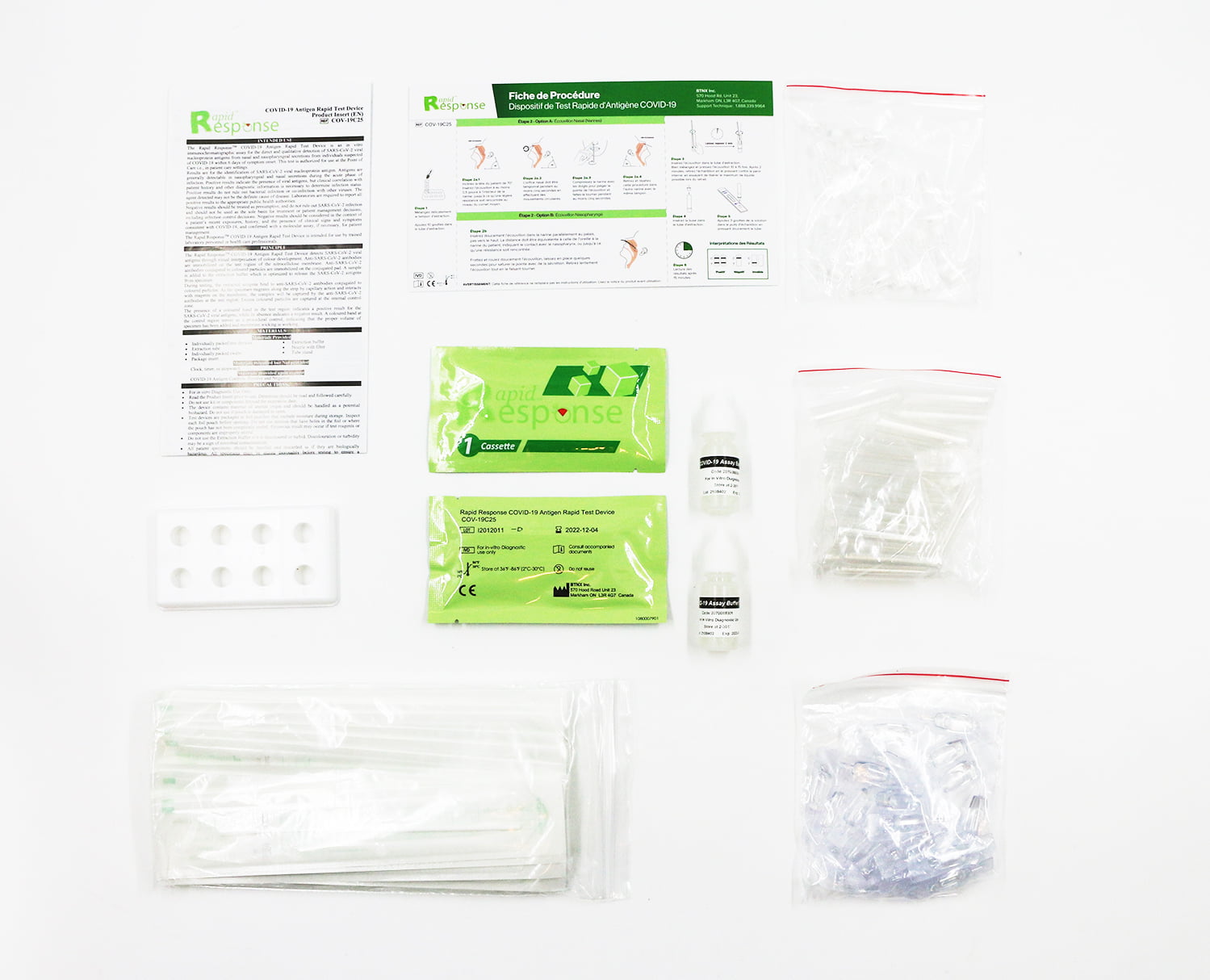
The Rapid Response™ COVID-19 Antigen Rapid Test Device is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal and nasopharyngeal secretions from individuals suspected of COVID-19 within 6 days of symptom onset and from individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection, when tested twice over two (or three) days with at least 24 hours (and no more than 36 hours) between tests. This test is authorized for use at the Point of Care i.e., in patient care setting.
- Fast identification of potentially contagious individuals
- Test results in 15 minutes
- Deploy at large scale at point of care
- Can be used in a wide variety of non-laboratory settings
- No special/additional instruments required
- 25 tests per kit
Stored Temperatures: 2-30 degrees Celsius
Shelf Life: 12 months
Health Canada Authorization: Point of Care
- Authorization Reference Number: 321669
- Device ID: 1029056
- Device Identifier: COV-19C25
![]()